Introduction:
Medical device manufacturing plays a pivotal role in the healthcare industry, driving innovation, improving patient care, and revolutionizing treatment options. With rapid advancements in technology, medical devices have become more sophisticated, precise, and interconnected. This comprehensive article explores the cutting-edge advancements in medical device manufacturing, highlighting their impact on healthcare technologies and the potential for enhancing patient outcomes.
- Miniaturization and Wearable Devices
Miniaturization and the development of wearable medical devices have transformed healthcare by providing portable, non-invasive, and patient-centric solutions. These devices offer numerous benefits in terms of monitoring, diagnostics, and treatment. Here are key aspects of miniaturization and wearable devices:
a. Portability and Convenience: Miniaturization has made it possible to develop lightweight and compact medical devices that can be easily worn or carried by patients. This portability allows for continuous monitoring and data collection, even in non-clinical settings. Wearable devices enable patients to go about their daily activities while still receiving vital health information.
b. Continuous Monitoring and Data Collection: Wearable medical devices can track various health parameters continuously, providing real-time data on vital signs, physical activity, sleep patterns, and more. This continuous monitoring offers valuable insights into a patient’s health status, enabling healthcare providers to detect and address potential issues promptly. By capturing long-term data trends, wearable devices contribute to better disease management and prevention.
c. Remote Patient Monitoring: Wearable devices, when coupled with wireless connectivity, facilitate remote patient monitoring. Patient data collected by wearable devices can be transmitted to healthcare providers in real-time, allowing for timely interventions and proactive care management. Remote patient monitoring improves patient outcomes, reduces hospital readmissions, and enhances patient satisfaction by minimizing the need for frequent clinic visits.
d. Personalized and Adaptive Healthcare: Wearable devices enable personalized healthcare by tailoring interventions and treatment plans based on individual patient data. These devices can track patient-specific information, such as medication adherence, physical activity levels, and physiological responses, providing valuable insights to healthcare providers. This personalized approach enhances treatment effectiveness, promotes patient engagement, and improves patient compliance with medical recommendations.
e. Empowering Patient Engagement: Wearable devices empower patients to actively participate in their healthcare journey. By providing access to real-time health data, these devices enable patients to monitor their progress, make informed lifestyle choices, and take preventive measures. Wearable devices foster a sense of ownership and empowerment, leading to increased patient engagement and improved health outcomes.
f. Integration with Digital Health Ecosystem: Miniaturized and wearable medical devices seamlessly integrate with the broader digital health ecosystem. They can synchronize with smartphone applications and cloud-based platforms, allowing for data storage, analysis, and sharing. Integration with digital health platforms enables healthcare providers to access comprehensive patient data, facilitates collaboration, and supports data-driven decision-making.
The miniaturization of medical devices and the development of wearable technologies have revolutionized healthcare by enabling continuous monitoring, personalized care, and patient engagement. These devices offer portability, convenience, and real-time data collection, enhancing disease management and promoting preventive healthcare practices.
- Telemedicine and Remote Patient Monitoring
Telemedicine and remote patient monitoring have emerged as transformative technologies in healthcare, facilitated by advancements in medical device manufacturing. These technologies enable healthcare providers to remotely monitor patients’ health and provide virtual healthcare services, revolutionizing the way healthcare is delivered. Here are key aspects of telemedicine and remote patient monitoring:
a. Virtual Consultations: Telemedicine platforms facilitated by medical devices enable healthcare providers to conduct virtual consultations with patients. Through audio and video communication, healthcare professionals can remotely assess patients, diagnose conditions, and provide treatment recommendations. Virtual consultations offer convenience, particularly for patients in remote or underserved areas, and minimize the need for in-person visits, saving time and resources for both patients and healthcare providers.
b. Real-Time Health Monitoring: Medical devices equipped with remote patient monitoring capabilities enable healthcare professionals to remotely track patients’ vital signs, symptoms, and other health parameters in real-time. This continuous monitoring allows for early detection of changes in health status, timely interventions, and proactive management of chronic conditions. Remote patient monitoring improves patient outcomes, reduces hospital admissions, and enhances patient satisfaction by providing personalized care from the comfort of their own homes.
c. Chronic Disease Management: Telemedicine and remote patient monitoring play a vital role in managing chronic diseases. Patients with conditions such as diabetes, hypertension, or heart disease can use medical devices to monitor their health status regularly. The collected data is transmitted to healthcare providers who can review it remotely, make necessary adjustments to treatment plans, and provide timely guidance to patients. This approach improves disease management, reduces complications, and enhances the quality of life for individuals with chronic conditions.
d. Access to Specialized Care: Telemedicine bridges the geographical gap between patients and specialized healthcare professionals. Medical device manufacturers have developed technologies that facilitate remote consultations with specialists. Patients can access expert opinions and guidance without the need for extensive travel or long wait times. Telemedicine enables patients in remote or underserved areas to receive specialized care, leading to improved health outcomes and a more equitable healthcare system.
e. Postoperative Care and Rehabilitation: Telemedicine and remote patient monitoring are valuable in postoperative care and rehabilitation. Medical devices can be used to monitor patients’ progress after surgery, track their recovery, and provide guidance on rehabilitation exercises or medication adherence. Healthcare providers can remotely assess patients’ healing process, identify any complications, and intervene when necessary. Telemedicine enhances postoperative care efficiency, reduces hospital visits, and supports patients’ recovery in the comfort of their own homes.
f. Health Data Integration: Telemedicine and remote patient monitoring devices are designed to integrate with electronic health records and other healthcare systems. This integration allows for seamless sharing of patient data between healthcare providers, ensuring continuity of care and efficient information exchange. Health data integration supports data-driven decision-making, enhances care coordination, and improves the overall healthcare experience for patients.
Telemedicine and remote patient monitoring have revolutionized healthcare by providing access to care regardless of geographical barriers, enhancing continuous monitoring, and improving communication between patients and healthcare providers. By embracing these technologies, medical device manufacturers contribute to the advancement of patient-centered care and the optimization of healthcare delivery.
- Robotics and Minimally Invasive Surgery
Robotics and minimally invasive surgical techniques have transformed the field of surgery, offering numerous benefits such as precision, reduced invasiveness, and faster recovery times. These advancements in medical device manufacturing have revolutionized surgical procedures and patient outcomes. Here are key aspects of robotics and minimally invasive surgery:
a. Robotic-Assisted Surgery: Robotic systems designed for surgical applications offer enhanced precision, dexterity, and control. Surgeons can utilize robotic-assisted technology to perform complex procedures with increased accuracy, even in confined spaces. Robotic systems consist of robotic arms, equipped with specialized surgical instruments and a high-definition camera, controlled by the surgeon through a console. This technology allows for a greater range of motion, improved visualization, and precise movements during surgical interventions.
b. Minimally Invasive Techniques: Minimally invasive surgery involves making small incisions, often less than an inch, to access the surgical site. Through these small incisions, surgical instruments and a tiny camera (laparoscope) are inserted, allowing the surgeon to visualize and operate inside the body. Minimally invasive techniques offer numerous advantages, including reduced postoperative pain, faster recovery, shorter hospital stays, and minimized scarring. These techniques are employed in various surgical specialties, such as gynecology, urology, orthopedics, and general surgery.
c. Benefits for Patients: Robotics and minimally invasive surgery benefit patients in several ways. The precision and accuracy provided by robotic systems minimize the risk of complications and improve surgical outcomes. Patients experience reduced blood loss, less postoperative pain, decreased risk of infection, and shorter recovery times compared to traditional open surgery. Minimally invasive techniques result in smaller scars, improved cosmetic outcomes, and a quicker return to daily activities, enhancing overall patient satisfaction.
d. Surgeon Advantages: Robotics and minimally invasive techniques also offer advantages for surgeons. Robotic-assisted surgery provides enhanced visualization and precise instrument control, enabling surgeons to perform complex procedures with greater ease. The ergonomic design of robotic systems reduces surgeon fatigue, leading to improved surgical precision and patient safety. Minimally invasive techniques require specialized training for surgeons to develop the necessary skills, but once mastered, they can enhance surgical proficiency and expand the range of procedures that can be performed.
e. Expanding Applications: Robotics and minimally invasive techniques continue to advance, expanding their applications across various surgical specialties. These techniques are utilized in procedures such as prostatectomies, hysterectomies, cholecystectomies, hernia repairs, joint replacements, and more. As technology evolves, robotic systems are becoming increasingly versatile, enabling surgeons to address complex anatomical challenges and perform intricate procedures with greater precision.
f. Continuous Innovation: Medical device manufacturers are continuously innovating to further improve robotics and minimally invasive surgical technologies. Advancements in robotic systems focus on enhancing dexterity, incorporating haptic feedback, improving imaging capabilities, and enabling remote surgery. Manufacturers also strive to make these technologies more accessible, cost-effective, and compatible with existing surgical infrastructures. Continuous innovation ensures that robotics and minimally invasive surgery continue to revolutionize healthcare and offer better treatment options for patients.
Robotics and minimally invasive surgery have revolutionized surgical practices, enabling surgeons to perform complex procedures with greater precision and patients to benefit from reduced invasiveness and improved outcomes. These advancements in medical device manufacturing continue to shape the future of surgery, advancing patient care and improving quality of life.
- Artificial Intelligence and Machine Learning
Artificial Intelligence (AI) and Machine Learning (ML) technologies have made significant contributions to the field of healthcare, offering advanced data analysis, predictive modeling, and decision-making capabilities. In medical device manufacturing, AI and ML are driving innovation, transforming diagnostics, treatment planning, and patient care. Here are key aspects of AI and ML in healthcare:
a. Data Analysis and Pattern Recognition: AI and ML algorithms can analyze vast amounts of healthcare data, including medical records, imaging scans, genomics data, and sensor data from wearable devices. These algorithms are designed to identify patterns, trends, and anomalies that might not be easily detectable by human analysis. By leveraging AI and ML, healthcare providers can make more accurate diagnoses, predict disease progression, and tailor treatment plans to individual patients’ needs.
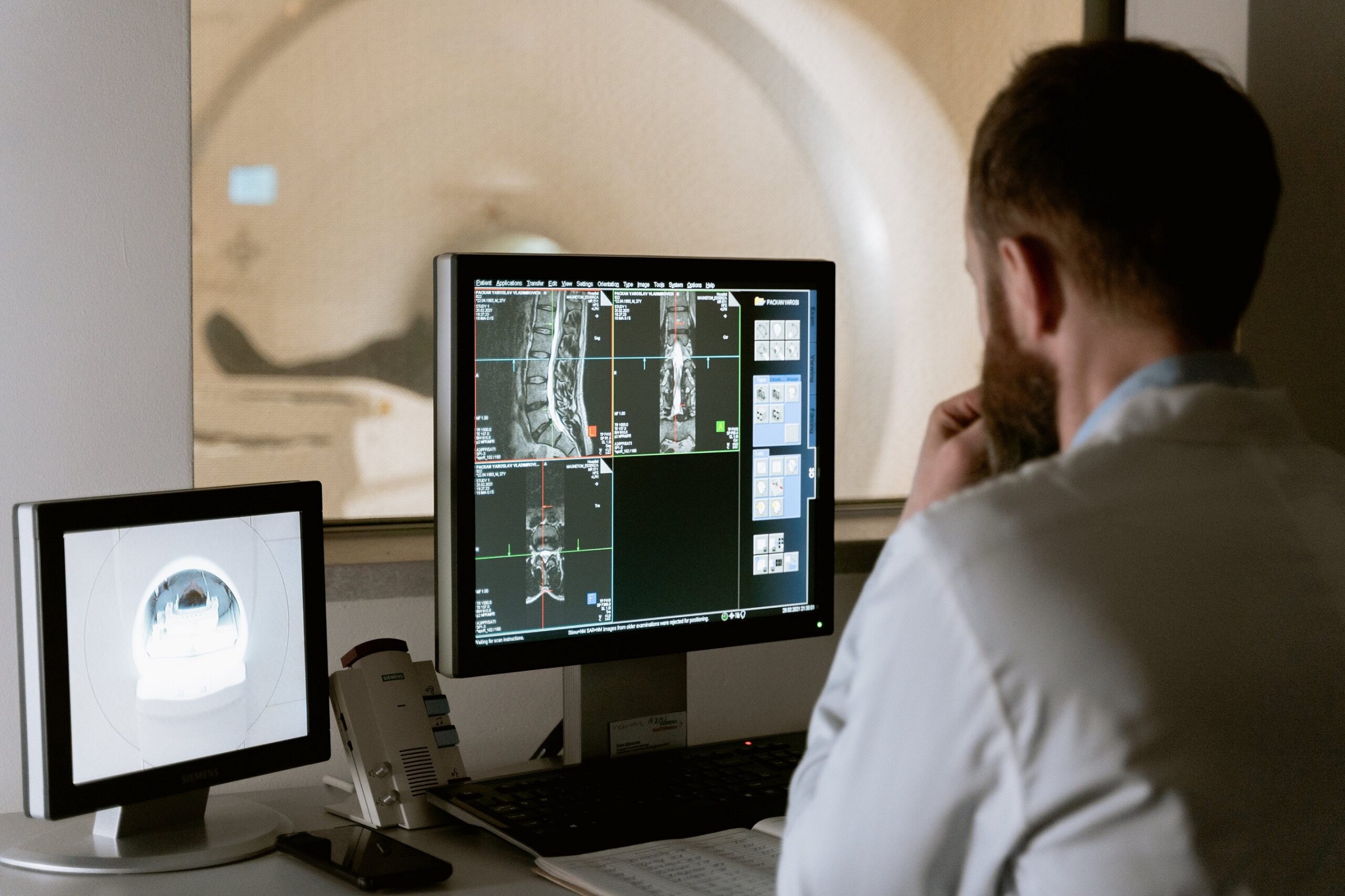
b. Medical Imaging and Diagnostics: AI and ML algorithms are revolutionizing medical imaging and diagnostics. These technologies can analyze medical images, such as X-rays, CT scans, and MRIs, to detect and classify abnormalities with high accuracy. AI-powered image analysis helps radiologists and clinicians in early detection of diseases, such as cancer, cardiovascular conditions, and neurological disorders. By automating image interpretation, AI and ML algorithms improve diagnostic efficiency, reduce human error, and enhance patient outcomes.
c. Predictive Analytics and Risk Assessment: AI and ML models can utilize patient data to predict health outcomes and assess the risk of developing certain conditions. By analyzing historical data, these models can identify risk factors, predict disease progression, and estimate the likelihood of complications. Predictive analytics and risk assessment tools empower healthcare providers to intervene early, implement preventive measures, and personalize treatment plans based on individual patient profiles.
d. Treatment Planning and Precision Medicine: AI and ML technologies enable personalized treatment planning and precision medicine approaches. These technologies can analyze patient-specific data, including genetic information, medical history, and treatment response, to guide treatment decisions. AI and ML models can suggest optimal treatment options, identify potential adverse reactions to medications, and assist in designing targeted therapies. Precision medicine, driven by AI and ML, ensures that patients receive tailored treatments that maximize efficacy and minimize side effects.
e. Remote Monitoring and Predictive Maintenance: AI and ML algorithms can be applied to remote patient monitoring systems and medical devices themselves. By analyzing real-time patient data, such as vital signs, medication adherence, and activity levels, these algorithms can identify early warning signs of deterioration, enabling timely interventions. Additionally, AI-powered predictive maintenance can monitor the performance of medical devices, detecting potential malfunctions or failures before they occur. This proactive approach ensures the continuous and reliable operation of medical devices, minimizing downtime and optimizing patient care.
f. Ethical Considerations and Data Privacy: With the increased use of AI and ML in healthcare, ethical considerations and data privacy become paramount. Medical device manufacturers must adhere to strict regulations and guidelines to ensure patient data confidentiality, consent, and responsible use of AI and ML technologies. Ethical considerations involve transparency in algorithmic decision-making, fairness in treatment recommendations, and responsible handling of patient data throughout the product lifecycle.
AI and ML technologies are transforming healthcare by enabling advanced data analysis, accurate diagnostics, personalized treatment planning, and predictive modeling. Medical device manufacturers play a crucial role in developing AI and ML-driven solutions that empower healthcare providers and improve patient outcomes.
- 3D Printing and Customized Medical Devices
3D printing, also known as additive manufacturing, has emerged as a groundbreaking technology in the field of medical device manufacturing. It enables the production of highly customized medical devices and components with exceptional precision and complexity. Here are key aspects of 3D printing and its impact on the healthcare industry:
a. Customization and Personalization: 3D printing allows for the creation of personalized medical devices tailored to the unique anatomical needs of individual patients. By leveraging patient-specific imaging data, such as CT scans or MRI images, medical devices can be precisely designed and manufactured to fit the patient’s anatomy with utmost accuracy. Customized devices offer improved functionality, comfort, and better clinical outcomes.
b. Complex and Intricate Designs: 3D printing enables the fabrication of intricate and complex geometries that would be challenging or impossible to achieve through traditional manufacturing methods. This includes complex structures, lattice patterns, and internal channels within medical devices. Such designs can optimize device performance, promote better integration with tissues, and enhance patient safety.
c. Rapid Prototyping and Iterative Design: 3D printing allows for rapid prototyping, significantly reducing the time and cost involved in the product development cycle. Medical device manufacturers can quickly produce physical prototypes, evaluate their performance, and make necessary design modifications. This iterative design process facilitates faster innovation and accelerates the time to market for new medical devices.
d. Surgical Guides and Implants: 3D printing is widely utilized in the production of surgical guides and patient-specific implants. Surgical guides are created based on preoperative imaging data and assist surgeons in precise surgical planning and execution. Patient-specific implants, such as cranial implants, orthopedic implants, and dental implants, are designed and 3D printed to perfectly match the patient’s anatomy, improving surgical outcomes and patient satisfaction.
e. Biofabrication and Tissue Engineering: 3D printing plays a crucial role in the field of tissue engineering and regenerative medicine. Biofabrication techniques utilize 3D printing to create scaffolds or frameworks that mimic the structure of human tissues or organs. These scaffolds can be populated with patient-derived cells, encouraging tissue regeneration and the growth of functional replacement tissues. 3D bioprinting holds tremendous potential in the development of patient-specific organs, such as liver tissue, skin grafts, and cartilage implants.
f. Supply Chain Optimization: 3D printing offers opportunities for supply chain optimization in medical device manufacturing. It allows for on-demand production, eliminating the need for large inventories and reducing lead times. Customized medical devices can be manufactured locally, reducing transportation costs and ensuring timely access to critical healthcare products. Additionally, 3D printing can facilitate distributed manufacturing, enabling remote or underserved areas to produce medical devices locally and address specific healthcare needs.
g. Regulatory Considerations: With the increasing adoption of 3D printing in medical device manufacturing, regulatory considerations are essential. Medical device manufacturers must comply with regulatory requirements and demonstrate the safety, efficacy, and quality of 3D-printed medical devices. Regulatory agencies are continuously evolving guidelines to ensure the appropriate use of 3D printing technology while safeguarding patient safety.
3D printing is revolutionizing medical device manufacturing by enabling customization, complex designs, rapid prototyping, and the production of patient-specific implants. The technology holds immense potential for advancing personalized medicine, regenerative therapies, and optimizing the healthcare supply chain.
- Data Security and Privacy
With the increasing use of interconnected devices and digital technologies in healthcare, ensuring data security and privacy has become paramount. Medical device manufacturers are focused on implementing robust security measures to safeguard patient information and protect against unauthorized access. Here are key aspects of data security and privacy in medical device manufacturing:
a. Patient Data Protection: Medical devices collect and process sensitive patient data, including personal health information, diagnostic results, and treatment records. It is crucial to ensure the confidentiality, integrity, and availability of this data to protect patient privacy and comply with regulatory requirements. Medical device manufacturers implement encryption techniques, access controls, and secure communication protocols to safeguard patient data throughout its lifecycle.
b. Secure Connectivity: Connected medical devices, such as wearable devices, remote monitoring systems, and implantable devices, rely on secure connectivity to transmit data to healthcare providers or cloud-based platforms. Robust encryption and authentication mechanisms are employed to establish secure connections and prevent unauthorized access or tampering of data during transmission. Secure connectivity ensures the privacy and integrity of patient information, even in wireless or remote settings.
c. Vulnerability Management: Medical device manufacturers proactively address potential vulnerabilities by conducting comprehensive risk assessments, vulnerability testing, and penetration testing. By identifying and addressing security vulnerabilities, manufacturers can minimize the risk of unauthorized access, data breaches, and system compromises. Regular software updates and patches are released to mitigate known vulnerabilities and ensure the ongoing security of medical devices.
d. User Authentication and Authorization: Medical devices incorporate strong user authentication mechanisms to ensure that only authorized personnel can access and interact with the device and its associated data. This may involve password-based authentication, biometric authentication, or multifactor authentication methods. User authorization controls are implemented to limit access rights based on the user’s role and responsibilities, ensuring that only authorized individuals can perform specific actions or access sensitive data.
e. Regulatory Compliance: Medical device manufacturers must comply with regulatory frameworks and standards that govern data security and privacy in healthcare. Regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in the European Union impose strict requirements for protecting patient data. Medical device manufacturers adhere to these regulations, implement security controls, and undergo audits to demonstrate compliance and maintain the trust of healthcare providers and patients.
f. Incident Response and Data Breach Management: Despite preventive measures, the possibility of a data breach or security incident cannot be entirely eliminated. Medical device manufacturers establish robust incident response plans to detect, respond to, and recover from security incidents promptly. This involves proactive monitoring of device and network activity, rapid incident notification, and coordinated response efforts to mitigate the impact of a breach and safeguard patient data.
Data security and privacy are critical considerations in medical device manufacturing. By implementing robust security measures, ensuring secure connectivity, and complying with regulatory requirements, medical device manufacturers protect patient data and maintain the trust of healthcare providers and patients.
- Regulatory Considerations and Standards
The medical device industry operates within a regulatory framework to ensure the safety, efficacy, and quality of medical devices. Regulatory considerations and adherence to standards play a vital role in medical device manufacturing. Here are key aspects of regulatory considerations and standards:
a. Regulatory Agencies: Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in the European Union, and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, oversee the approval, marketing, and post-market surveillance of medical devices. These agencies establish guidelines, review submissions, and conduct inspections to ensure compliance with regulatory requirements.
b. Pre-Market Approval: Medical devices typically undergo a rigorous pre-market approval process before they can be commercially distributed. This process involves submitting detailed technical documentation, clinical data (if required), and evidence of safety and performance to the regulatory agency. The agency reviews the submission and determines whether the device meets the necessary regulatory standards for market approval.
c. Quality Management Systems: Medical device manufacturers are required to implement robust quality management systems (QMS) to ensure consistent product quality and compliance with regulatory requirements. International standards, such as ISO 13485, outline the requirements for a QMS specific to medical devices. QMS encompasses various aspects, including document control, risk management, supplier management, and post-market surveillance.
d. Post-Market Surveillance: Once a medical device is approved and on the market, ongoing post-market surveillance is necessary to monitor its performance, safety, and any adverse events. Medical device manufacturers have processes in place to track and analyze post-market data, including feedback from healthcare professionals and patients. This information helps identify potential risks, implement corrective actions, and ensure continued compliance with regulatory requirements.
e. Labeling and Instructions for Use: Medical devices must have accurate and comprehensive labeling and instructions for use. This includes information on device identification, indications for use, warnings, precautions, and instructions for proper device handling, storage, and maintenance. Clear and concise labeling ensures that healthcare professionals and patients have the necessary information to use the device safely and effectively.
f. Global Harmonization: Medical device regulatory frameworks are continuously evolving to achieve global harmonization. Efforts are made to align regulatory requirements across different regions, streamlining the approval process for medical devices and promoting international trade. Harmonization initiatives, such as the International Medical Device Regulators Forum (IMDRF), facilitate collaboration among regulatory agencies to establish common guidelines and standards.
g. Unique Device Identification (UDI): Regulatory agencies have introduced UDI systems to uniquely identify and track medical devices throughout their lifecycle. UDI implementation involves assigning a unique identifier to each device, which helps with product traceability, recall management, and post-market surveillance. Medical device manufacturers must comply with UDI requirements and include the UDI on device labels and packaging.
h. Adverse Event Reporting: Medical device manufacturers have a responsibility to report adverse events associated with their devices to the regulatory agency. Adverse event reporting allows for the identification of potential safety issues and facilitates timely actions to mitigate risks. Manufacturers have established processes to collect, evaluate, and report adverse events as per regulatory requirements.
Regulatory considerations and adherence to standards are essential in medical device manufacturing to ensure patient safety, product quality, and compliance with applicable regulations. Medical device manufacturers collaborate closely with regulatory agencies, implement robust quality management systems, and continuously monitor device performance to meet regulatory requirements.
Conclusion
Advancements in medical device manufacturing have the power to transform healthcare, improve patient outcomes, and drive innovation in the industry. From miniaturized wearables to AI-powered diagnostics and robotic-assisted surgery, these innovations are reshaping the way healthcare is delivered. Embracing these advancements requires a collaborative effort between manufacturers, healthcare professionals, regulatory bodies, and patients. By leveraging cutting-edge technologies responsibly and adhering to robust quality and safety standards, the healthcare industry can harness the full potential of medical device manufacturing to improve lives and shape the future of healthcare.
I have noticed that of all types of insurance, health insurance coverage is the most dubious because of the struggle between the insurance company’s duty to remain profitable and the client’s need to have insurance cover. Insurance companies’ earnings on wellness plans are extremely low, so some organizations struggle to make a profit. Thanks for the strategies you share through this website.